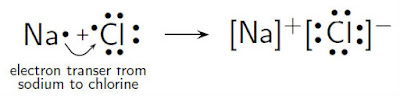- Saponification: Alkaline hydrolysis of an ester where the ester is boiled with sodium hydroxide, NaOH or potassium hydroxide, KOH solution to produce an alcohol and a sodium or potassium salt of a carboxylic acid.
- Soap is not an effective cleansing agent in hard water but is only effective in soft water. Detergent is an effective cleansing agent in both hard water and soft water.
- Soft water: Water that does not contain mineral salts such as magnesium salt or calcium salts.
- Hard water: Water that contain mineral salts.
- Food additives are substances added to food to preserve flavor or enhance its taste and appearance. Types of additives are preservatives, antioxidants, flavourings, stabilizers, thickening agents and food dyes (colourings).
- Medicines: a compound or preparation used for the treatment or prevention of disease, especially a drug or drugs taken by mouth. Types of modern medicines are analgesics, antibiotics, stimulants, antidepressant and antipsychotic.
SPM Form 5: Chemicals for Consumers (Checklist)
SPM Form 5: Carbon compounds (Checklist)
- Organic compounds: Carbon-containing compounds that can be obtained from living things.
- Inorganic compounds: Non-carbon-containing compounds that can be obtained from non-living things.
- Hydrocarbons: Compounds that contain only carbon and hydrogen. In a saturated hydrocarbon, all the bonds are single bonds. Alkane is another name for a saturated hydrocarbon. Unsaturated hydrocarbons are a hydrocarbon that contains one or more double or triple bonds are an unsaturated hydrocarbon. There are three types of unsaturated hydrocarbons alkenes, alkynes, and aromatic hydrocarbons.
- Alkanes: Hydrocarbons having the general formula CnH2n+2, where n = 1, 2
- Alkenes: Hydrocarbons that contain one or more carbon-carbon double bonds. They have the general formula CnH2n.
- Alcohol: An organic compound containing the hydroxyl group -OH.
- Aldehydes: Compounds with a carbonyl functional group and the general formula RCHO, where R is an H atom, an alkyl, or an aromatic group.
- Carboxylic acids: Acids that contain the carboxyl group -COOH.
- Esters: Compounds that have the general formula R’COOR, where R’ can be H or an alkyl group or an aromatic group and R is an alkyl group or an aromatic group.
- Homologous series: A series of compounds in which each member differs from the next by a specific number and kind of atoms.
- Esterification: A reaction between an alcohol and a carboxylic acid in the presence of concentrated sulphuric acid as a catalyst through boiling to produce an ester and water. e.g. reaction of ethanol, C2H5OH with ethanoic acid, CH3COOH in the presence of concentrated sulphuric acid, H2SO4 produces ethyl ethanoate, CH3COOC2H5 which is an ester with a pleasant fragrant smell.
- Fats: Solid triester of glycerol and mostly saturated fatty acids.
- Vulcanization of rubber: A process whereby rubber is reacted with sulphur to enable the formation of cross-linkages by sulphur atoms between the rubber molecules through covalent bonds. Rubber can be vulcanized by dipping natural rubber sheets into disulphur dichloride solution in methylbenzene. Vulcanized rubber is hard whereas unvulcanized rubber is soft. Vulcanized rubber is more elastic than unvulcanized rubber.
- Coagulation of latex: A process of converting liquid latex to solid natural rubber by adding an acid. Coagulation of latex occurs rapidly when an acid is added to the latex. Latex does not coagulate when an alkali is added to the latex. Alkaline solutions contain hydroxide ions which neutralize the acid produced by bacteria. Hence, it prevents the latex from coagulating.
Indicators and their colours in acid and alkaline solution
- An indicator is substances that have distinctly different colors in acidic and basic media. Many indicators are dyes which have been extracted from natural sources, for example litmus.
- Methyl orange, a common indicator used in titrations is pink in an acid solution but changes to show a yellow colour in an alkaline solution.
- Phenolphthalein is pale-yellow crystals; soluble in alcohol, ether, and alkalis, insoluble in water; used as an acid-base indicator (carmine-colored to alkalis, colorless to acids) for titrations.
Electrolysis of molten lead (II) bromide
During the
electrolysis of molten lead(II) bromide, PbBr2, bromine gas, Br2
is released at the cathode while the lead metal is formed at the anode.
Pb2+
and Br- ions are present in the molten lead(II) bromide, PbBr2. At the anode, bromide ions, Br-
are discharged by donating electrons to form bromine molecules, Br2.
At the cathode, each lead(II) ion, Pb2+ is discharged by accepting
two electrons to form a lead atom.
Formation of hydrogen molecules
Hydrogen atom has one
valency. To become stable with hydrogen atom, it needs one more electron, When
2 hydrogen atoms join, they share their electrons, on which, the share becomes
2 electrons, which is now a noble gas configuration, being shared between these
2 atoms.
H – H single bond [they
share an electron pair (2 electrons)].
Summary of Laws of Thermodynamics
- First Law: In any process, the total energy of the universe remains constant.
Universe = system +
surroundings.
- Second Law: In any spontaneous process, the overall entropy of the universe increases.
- Third Law: As temperature approaches absolute zero, the entropy of a system approaches a constant minimum.
Rules for figuring out oxidation numbers
- Atoms in elemental form have an oxidation number of zero.
- Single-atom (monoatomic) ions have an oxidation number equal to their charge.
- In a neutral compound, oxidation numbers add up to zero. In a charged compound, oxidation numbers add up to the compound’s charge.
- In compounds, oxygen usually has an oxidation number of –2, in which its oxidation number is –1.
- In compounds, hydrogen has an oxidation number of +1 when it bonds to nonmetals and an oxidation number of –1 when it bonds to metals.
- In compounds
Group 1 atoms (alkali
metals) have oxidation number +1.
Group 2 atoms (alkaline
earth metals) have oxidation number +2.
Group 3 atoms have oxidation
number +3.
Group 17 atoms (halogens)
usually have oxidation number –1.
Explain why the melting and boiling points of argon are higher than helium?
The atomic size of argon is
bigger than helium. Thus, the forces of attraction between argon atoms are
stronger than the forces of attraction between helium atoms. As a result, more
heat energy is required to overcome the stronger forces of attraction between
argon atoms. Hence, the melting and boiling points of argon are higher than
helium.
Why is helium gas used in airships while argon gas used in electric bulbs?
- Helium gas is suitable to fill airships because it is very light and non-flammable.
- Argon gas is suitable to use in electric bulbs because it is chemically inert. Hence, the hot tungsten filament in the electric bulb does not react with it.
Explain why water has a high specific heat capacity
Water has a high specific
heat capacity because a large amount of thermal energy needs to be absorbed in
order to break the hydrogen bonds among water molecules before the individual
water molecules can begin to move about more freely and therefore, causing an
increase in temperature.
Explain why ice floats
At O˚C, water molecules
become locked into a crystalline lattice structure; each water molecule bonded
to four other molecules through hydrogen bonding. This is the solid state of
water called ice. The hydrogen bonds keep the water molecules far apart enough
to give ice a density of about 10% less than that of liquid water. The crystalline
structure provides a larger volume for the same amount of liquid water molecules.
Therefore, ice is less dense compared to liquid water and ice floats on liquid
water.
Describe the variation in melting points and electrical conductivities of the Period 3 elements from sodium to chlorine in terms of their structure and bonding.
Sodium, magnesium and
aluminium have giant metallic structures with strong metallic bonds. They have
relatively high melting point. Silicon has a giant covalent structure with
strong covalent bonds. This accounts for its exceptionally high melting point.
The rest are simple molecules with weak intermolecular van der Waals forces.
The strength of the van der Waals force and the melting point increases in the
order: Ar ˂ Cl2 ˂ P4 ˂ S8. Sodium, magnesium
and aluminium have mobile electrons. They are conductors. Silicon is a
metalloid. The energy gap between the conduction band and the valence bond is
small. It is a semi-conductor. Phosphorous, sulphur, chlorine and argon do not
have mobile electrons. They are non-conductors.
State the general properties of ionic compounds and covalent compounds.
General properties of ionic
compounds:
- Solid at room conditions.
- High melting point and boiling point.
- Soluble in water but insoluble in non-polar solvents.
- Do not conduct electricity in the solid state.
- Conduct electricity in molten state or in aqueous solutions.
General properties of
covalent compounds:
- Low boiling point and melting point.
- Insoluble in water but soluble in non-polar solvents.
- Do not conduct electricity, either in the solid or molten state.
Weak acids and weak bases
Weak acids
- Weak acids dissociate only slightly in an aqueous solution to produce a low concentration of H3O+.
- % dissociation less than 100% or α ˂˂ 1.
Weak bases
- Weak bases dissociate only slightly in an aqueous solution to produce a low concentration of OH-.
- % dissociation less than 100% or α ˂˂ 1.
Strong acids and strong bases
Strong acid
- Strong acids dissociate completely in an aqueous solution to produce high concentration of H3O+.
- 100% ionization or 100% dissociation or α = 1
Strong base
- Strong bases dissociate completely in an aqueous solution to produce high concentration of OH-.
- 100% ionization or 100% dissociated or α=1
Structures and properties of metallic bonds
- Metals can be bent (ductile) and can be stretched (malleable) because the layers of atoms in metals slide over each other when force is applied but will not break due to attractive force between electrons and metal ions.
- Metals conduct electricity as it has free electrons which carry current.
- Metals conduct heat as it has free electrons which gains energy when heated and moves faster to collide with metal atoms, releasing heat in collisions.
- Metals have high melting and boiling points because the bonds between metals are very strong. Hence very high heat energy needed to break the bonds.
Ionic bonds versus Covalent bonds
- In ionic bonds, electron pairs are shared between atoms. In covalent bonds, atoms are electrostatically attracted towards each other.
- Ionic bonds occur through the interaction between cations and anions. Covalent bonds occur through the interaction of neutral atoms.
- Ionic bonds are the strongest type of chemical bond. Covalent bonds are quite weak.
- Metallic elements tend to form ionic bonds. Non-metallic elements tend to form covalent bonds.
Why ionic compounds have high boiling point than covalent compounds?
- Ionic compounds consist entirely of ions. The opposite charged ions are held close to one another by very strong electrostatic attraction, known as ionic bonds. Hence large amount of energy needed to break ionic bonds. Therefore high boiling points.
- Covalent compounds consist entirely of molecules as they are formed by sharing of electrons. Forces between molecules are very weak. Only small amount of energy needed to break binds. Therefore low boiling points.
Two factors that influence the changes of atomic radii in the Periodic Table
Two factors that influence
the changes of atomic radii in the Periodic Table are:
- Effective nuclear charge experienced by the valence electrons: Electrons around the nucleus experience different nucleus attraction. Those electrons closer to the nucleus experience a greater attraction than those that are farther away. The actual nuclear charge experienced by an electron is called the effective nuclear charge, Zeff. Effective nuclear charge increase, nucleus attraction stronger, atomic radii decrease. Across the period, the effective nuclear charge increases as proton number increase. As a result, the attraction between the nucleus and valence electrons become stronger, causing the atomic radius to decrease.
- The principal quantum number, n, of the valence electrons: As going down a group, the number of shells increases, more inner electrons are present to shield the valence electrons from the nucleus. The valence electrons are farther from the nucleus. Thus, the attraction between the nucleus and valence electrons decreases, therefore, the atomic radius increase. Down a group, the atomic radius increases because of the increasing principal quantum number (n) of the valence electron.
Properties of covalent compounds
- It is a hard solid because it consists of many strong covalent bonds between atoms. This property makes it suitable as abrasives.
- It has very high melting and boiling points.
- It does not conduct electricity (except graphite) because there are no free electrons in covalent bonds since they are used to form bonds; hence electrons are in fixed positions. To conduct electricity, there must be free electrons.
- All covalent structures are insoluble in water.
Formation of Oxygen gas
An O atom has 6 valency and
needs 2 electrons, each, to form a noble gas configuration. Hence, each share
the amount of electrons each short of, in this case – 2 electrons, to form
stable molecule. The contribution hence now becomes 4 electrons and what left
on each oxygen atom are 4 electrons. We combine each 4 electrons on oxygen atom
with the 4 electrons shared and hence we get 8 valency for each oxygen atom – a
noble gas configuration!
Formation of Chlorine gas
Cl atom has 7 valency and
needs one electron, each, to form a noble gas configuration between two Cl
atoms. Hence they share an electron each to hence share 2 electrons between the
atoms. Hence, each Cl atom now has 8 valency which is a noble gas
configuration.
Properties of ionic compounds
- Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arranged in straight rows in strong ionic bonds.
- Ionic compounds have very high melting points and boiling points.
- The strong forces holding ionic compounds prevent them to evaporate easily. Hence, ionic compounds have no smell.
- Solid ionic compounds don’t conduct electricity but they do when they are aqueous or molten. This is because in liquid/aqueous state the ions which conduct electricity are free to move. In solids, these ions are fixed in place.
- Ionic compounds are soluble in water but insoluble in organic compounds. This is because the ions attract water molecules which disrupt the crystal structure, causing them separate & go into solution. Vice versa is when in organic solvent.
Formation of magnesium floride
Magnesium atom loses two
electrons by transferring the electrons to fluorine atoms, one each, making
both stable. The loss of electron forms cation, Mg2+, as it loses 2
electrons, and the gain of electron forms anion, F-. The opposite
charges acquired by both ions attract to each other, forming a strong ionic
bond of MgF2.
Formation of NaCl
Sodium atom loses an
electron by transferring the electron to chlorine atom, making both stable. The
loss of electron forms cation, Na+, and the gain of electron forms
anion, Cl-. The opposite charges acquired by both ions attract to
each other, forming a strong ionic bond of NaCl.
Types of Food additives
- Thickening agents: substances added into food to reduce its amount of water so that the food is thickened. Examples: pectin, acacia gum, gelatin.
- Food dyes (colourings): substances added to food to add or restore the color of food in order to enhance its visual appeal and to match consumer expectations. Examples: Azo compounds such as tartrazine, triphenyl compounds such as brilliant blue FCF. Azo compounds are usually red, orange, brown or yellow in colour while triphenyl compounds are usually blue or green in color.
- Stabilisers: substances added into food to prevent an emulsion from being separated out. Examples: Lecithin, mono- and di-glycerides of fatty acids.
- Flavourings: substances added to food to improve the taste of food and to restore taste loss due to processing. Examples: Sugar, vinegar, common salt, aspartame, monosodium glutamate, synthetic essences such as penthyl ethanoate.
- Antioxidants: substances added to food to prevent oxidation by air that can cause rancid fats and browning of fruits. Examples: ascorbic acid, sodium citrate, tocopherol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT).
- Preservatives: Subtances added to food to slow down or to prevent the growth of microorganisms so that food can be kept for a longer period of time. Examples: sodium benzoate, sulphur dioxide, sodium nitrite, potassium sorbat.
Similarities and differences between alkanes and alkenes
SIMILARITIES:
DIFFERENCES:
Alkenes
- Both are insoluble in water
- Both are neutral
- Both burn in excess air to produce carbon dioxide and water.
DIFFERENCES:
Alkanes:
- General formula: CnH2n+2
- Saturated hydrocarbon
- Do not decolourise the purple acidified potassium manganate (VII) solution.
- Do not decolourise the reddish-brown bromine solution.
- Burn with a less sooty flame because the percentage by mass of carbon is lower than that of the corresponding alkenes.
Alkenes
- General formula: CnH2n
- Unsaturated hydrocarbon
- Decolourise the purple acidified potassium manganite (VII) solution.
- Decolourise the reddish-brown bromine solution.
- Burn with a more sooty flame because the percentage by mass of carbon is higher than that of the corresponding alkanes.
SPM Form 5: Rate of reaction (Checklist)
- Rate of reaction: The decrease in the amount of reactant used or the increase in the amount of product obtained in a given time. The rate of reaction measures how much product is formed in a certain time. Some reactions are slow, such as rusting, and some are fast, like burning.
- Average of reaction: The average value of the rate of reaction within a specified period of time.
- Rate of reaction at a given time: The actual rate of reaction at a specific time.
- Collision theory: A model that explains reaction rate as the result of particles colliding with a certain minimum energy.
- Effective collision: A collision in which the particles meet with sufficient energy and an orientation that allows them to react.
- The rate of reaction decreases with the increase of time.
- The rate of reaction increases as the total surface area of a solid reactant increases.
- The rate of reaction increases as the concentration of reactant increases.
- The rate of reaction increases as the temperature of a reactant increases.
- The rate of reaction increases when a positive catalyst is used and the rate of reaction increases when the amount of a positive amount used increases.
- Catalyst: A substance which alters the rate of a chemical reaction while it remains chemically unchanged at the end of the reaction.
Soap vs Detergent: Similarities and Differences
Similarities:
- Both are cleansing agents.
- Each ion of both soap and detergent consists of a long chain of hydrocarbon part which is hydrophobic and an ionic part which is hydrophilic.
- Both can reduce the surface tension of water, thus, allowing water to wet the surface.
- The cleansing actions of both soap and detergent enable oil droplets to disperse in water and form an emulsion that will not redeposit on the surface.
- Both are effective as cleansing agents in soft water.
Differences:
- Soap is biodegradable while detergent is not biodegradable (can cause environmental pollution).
- Detergent is an effective cleansing agent in hard water, soft water and in acidic solution but soap is only effective as a cleansing agent in soft water.
SPM Form 4: Salts (Checklist)
- Salts: An ionic compound formed when the hydrogen ion in an acid is replaced by a metal ion or an ammonium ion. Salts are formed as the product of an acid reaction with an alkali. Soluble salts dissolve in water. Insoluble salts do not dissolve in water.
- Recrystallization: A technique used to purify crystals of a soluble salt by carrying out the crystallization process again on these crystals.
- Precipitation reaction: A reaction which involves the reaction between two aqueous reactants to form an insoluble substance.
- Continuous variation method: An experiment which involves the reaction of a solution at fixed volume and another solution with volumes that varies uniformly.
- All carbonate salts are decomposed on heating to liberate carbon dioxide gas, CO2 except sodium carbonate, Na2CO3 and potassium carbonate, K2CO3.
- All nitrate salts are decomposed on heating to liberate nitrogen dioxide gas, NO2 and oxygen gas, O2 except sodium nitrate, NaNO3 and potassium nitrate, KNO3 which liberate oxygen gas, O2 only.
- The presence of carbonate ions can be confirmed by adding dilute acids.
- The presence of sulphate ions can be confirmed by adding acidified barium chloride, BaCl2 solution.
- The presence of chloride ions can be confirmed by adding acidified silver nitrate, AgNO3 solution.
- The presence of nitrate ions can be confirmed by adding dilute sulphuric acid, H2SO4 followed by iron (II) sulphate, FeSO4 solution and a little concentrated sulphuric acid, H2SO4.
- The identify of cation can be determined by using sodium hydroxide, NaOH solution and ammonia, NH2 solution except aluminium ion, Al3+ and lead (II) ion, Pb2+.
- Ammonium ion can be tested by heating with a strong alkali or adding Nessler’s reagent.
- Iron (II) ion and iron (III) ion can be tested by using potassium hexacyanoferrate (II), K4Fe(CN)6 solution, potassium hexacyanoferrate (III), K3Fe(CN)6 or potassium thiocyanate, KSCN solution.
- Lead (II) ions can be tested by using potassium iodide, KI solution.
SPM Form 4: Manufactured Substances in Industry (Checklist)
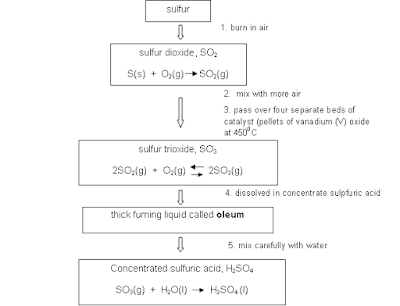
- Sulphuric acid (H2SO4) is a highly corrosive acid made from sulfur dioxide; widely used in the chemical industry.
- Contact process: the industrial process for making sulfuric acid.
- Ammonia (NH3) is colorless, pungent gas composed of nitrogen and hydrogen.
- Haber process: the process for making ammonia from nitrogen and hydrogen, in industry.
- Ammonium fertilizer: A salt that is prepared from the reaction between ammonia and an acid. Ammonium sulphate, (NH4)2 SO4 which is an ammonium fertilizer can be prepared from the reaction between ammonia, NH3 solution and sulphuric acid, H2SO4.
- Alloys: A mixture of two or more elements with certain fixed composition in which the major component is a metal. Alloy is harder than pure metal.
- Metal corrosion: The gradual destruction of a metal by reaction with its environmental. Iron rusts faster than steel. Stainless steel does not rust.
- Polymer: a compound containing very large molecules, formed by polymerization.
- Synthetic polymers are derived from petroleum oil, and made by scientists and engineers. Examples of synthetic polymers include nylon, polyethylene, polyester, Teflon, and epoxy.
- Natural polymers occur in nature and can be extracted and often water-based. Examples of naturally occurring polymers are silk, wool, DNA, cellulose and proteins.
- Glass: A homogeneous material with a random, liquid-like molecular structure.
- Ceramic is a hard, unreactive material that can withstand high temperatures, made by baking clay in a kiln; ceramics are non-conductors.
- A "composite" material is when two or more different materials are combined together to create a superior and unique material.
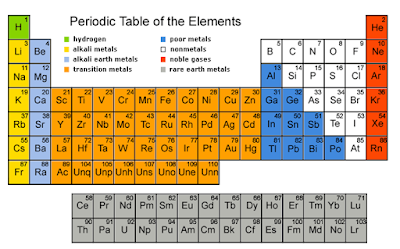
SPM Form 4: Periodic Table of elements (Checklist)
- Periodic Table: the table showing the elements in order of increasing proton number; similar elements are arranged in columns called groups.
- Group: A vertical column of elements in the Periodic table.
- Period: A horizontal row of the Periodic Table; its number tells you how many electron shells there are.
- Alkali metals: the Group I elements of the Periodic Table, which include lithium, sodium, potassium, rubidium, caesium and francium.
- Alkaline earth metals: the Group II elements of the Periodic Table, which include beryllium, magnesium, calcium, strontium, barium, and radium.
- Halogens: the Group VII elements of the Periodic Table, which include fluorine, chlorine, bromine, iodine and astatine.
- Noble gases: the Group 18 elements of the Periodic Table; they are called ‘noble’ or inert gases because they are so unreactive, which include helium, neon, argon, krypton, xenon and radon.
- Transition elements: the elements in the wide middle block of the Periodic Table (elements in group 3 to group 12).
- Metal: an element that shows metallic properties (for example conducts electricity, and forms positive ions)
- Non-metal - an element that does not show metallic properties: the non-metals lie to the right of the zig-zag line in the Periodic Table.
- Amphoteric oxide: An oxide that exhibits both acidic and basic properties.
- The atomic radius is a term used to describe the size of the atom.
- The ionization energy is the energy required to completely remove an electron from a gaseous atom or ion.
- Electron affinity reflects the ability of an atom to accept an electron. It is the energy change that occurs when an electron is added to a gaseous atom.
- Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. The higher the electronegativity of an atom, the greater its attraction for bonding electrons.
Uses of the noble gases
The noble gases are
unreactive or inert, which makes them safe to use. They also glow when a
current is passed through them at low pressure. These properties lead to
many uses:
- Helium is used to fill balloons and airships, because it is much lighter than air – and will not catch fire.
- Argon is used to provide an inert atmosphere. For example it is used: as filler in tungsten light bulbs and to protect metals that are being welded.
- Neon is used in advertising signs. It glows red, but the colour can be changed by mixing it with other gases.
- Krypton is used in lasers – for example for eye surgery – and in car headlamps.
- Xenon gives a light like bright daylight, but with a blue tinge. It is used in lighthouse lamps, lights for hospital operating rooms, and car headlamps.
Discuss the advantages and disadvantages of aspirin as a medicine
Advantages
- It is used to relieve pain such as arthritic pain, dental pain, etc where there is an inflammation involved.
- It is used to prevent thickening of blood and heart attack.
Disadvantages
- It is acidic and can cause internal bleeding and ulceration in the stomach.
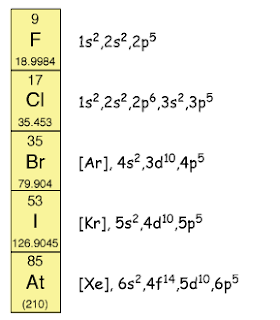
Explain the reactivity of halogens decreases when going down Group 17.
The atomic size of halogen
increases when going down Group 17. Thus, the outermost occupied shell becomes
further away from the nucleus. Hence, the strength of the nucleus to pull
another electron into the outermost occupied shell becomes weaker. Thus,
halogens are less reactive when going down Group 17.
Explain how the melting and boiling points change when going down the Group 1.
Melting and boiling points
of alkali metals decreases when going down Group 1. The atomic size of alkali
metals becomes bigger when going down the group 1. Hence, the forces between
alkali metal atoms are weaker when going down Group 1. Hence, less heat energy
is required to break the weaker forces.
Why noble gases chemically inert?
Helium has a duplet electron
arrangement whereas other noble gases have octet electron arrangements. These
electrons arrangements are very stable. Hence, atoms of noble gases do not
release electrons, accept electrons or share electrons with other atoms. Thus, they
are chemically inert.
Why is neon exists as a monoatomic gas?
- Neon is from the Noble Gases group on the periodic table.
- Neon has electron arrangement is 2.8 (valence electron is 8).
- Neon already achieves the octet electron arrangement.
- Neon does not need to donate the electron with other element.
- Therefore they do not gain or lose electrons making them exist as individual atoms.
- Neon exists as a monoatomic gas.
Kinetic Molecular Theory
- Matter consists of small particles that always move and collide among each other.
- The kinetic theory of matter stated the particles move freely when energy is absorb and move slowly when energy is release, then they are cooled.
- The kinetic molecular theory used to explain the change physical state of matter.
- Gas molecules are individual particles that travel in a straight-line random motion. This will continue until they collide or are acted upon by another force.
- Gas molecules continuously collide and transfer energy during these collisions. In an isolated sample of gas the net energy is conserved.
- The volume of the individual gas molecules is negligible compared to the volume they occupy.
- No forces of attraction are considered to exist between the gas molecules.