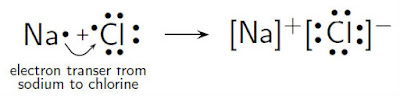Sodium atom loses an
electron by transferring the electron to chlorine atom, making both stable. The
loss of electron forms cation, Na+, and the gain of electron forms
anion, Cl-. The opposite charges acquired by both ions attract to
each other, forming a strong ionic bond of NaCl.