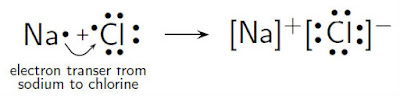- In ionic bonds, electron pairs are shared between atoms. In covalent bonds, atoms are electrostatically attracted towards each other.
- Ionic bonds occur through the interaction between cations and anions. Covalent bonds occur through the interaction of neutral atoms.
- Ionic bonds are the strongest type of chemical bond. Covalent bonds are quite weak.
- Metallic elements tend to form ionic bonds. Non-metallic elements tend to form covalent bonds.
Ionic bonds versus Covalent bonds
Why ionic compounds have high boiling point than covalent compounds?
- Ionic compounds consist entirely of ions. The opposite charged ions are held close to one another by very strong electrostatic attraction, known as ionic bonds. Hence large amount of energy needed to break ionic bonds. Therefore high boiling points.
- Covalent compounds consist entirely of molecules as they are formed by sharing of electrons. Forces between molecules are very weak. Only small amount of energy needed to break binds. Therefore low boiling points.
Two factors that influence the changes of atomic radii in the Periodic Table
Two factors that influence
the changes of atomic radii in the Periodic Table are:
- Effective nuclear charge experienced by the valence electrons: Electrons around the nucleus experience different nucleus attraction. Those electrons closer to the nucleus experience a greater attraction than those that are farther away. The actual nuclear charge experienced by an electron is called the effective nuclear charge, Zeff. Effective nuclear charge increase, nucleus attraction stronger, atomic radii decrease. Across the period, the effective nuclear charge increases as proton number increase. As a result, the attraction between the nucleus and valence electrons become stronger, causing the atomic radius to decrease.
- The principal quantum number, n, of the valence electrons: As going down a group, the number of shells increases, more inner electrons are present to shield the valence electrons from the nucleus. The valence electrons are farther from the nucleus. Thus, the attraction between the nucleus and valence electrons decreases, therefore, the atomic radius increase. Down a group, the atomic radius increases because of the increasing principal quantum number (n) of the valence electron.
Properties of covalent compounds
- It is a hard solid because it consists of many strong covalent bonds between atoms. This property makes it suitable as abrasives.
- It has very high melting and boiling points.
- It does not conduct electricity (except graphite) because there are no free electrons in covalent bonds since they are used to form bonds; hence electrons are in fixed positions. To conduct electricity, there must be free electrons.
- All covalent structures are insoluble in water.
Formation of Oxygen gas
An O atom has 6 valency and
needs 2 electrons, each, to form a noble gas configuration. Hence, each share
the amount of electrons each short of, in this case – 2 electrons, to form
stable molecule. The contribution hence now becomes 4 electrons and what left
on each oxygen atom are 4 electrons. We combine each 4 electrons on oxygen atom
with the 4 electrons shared and hence we get 8 valency for each oxygen atom – a
noble gas configuration!
Formation of Chlorine gas
Cl atom has 7 valency and
needs one electron, each, to form a noble gas configuration between two Cl
atoms. Hence they share an electron each to hence share 2 electrons between the
atoms. Hence, each Cl atom now has 8 valency which is a noble gas
configuration.
Properties of ionic compounds
- Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arranged in straight rows in strong ionic bonds.
- Ionic compounds have very high melting points and boiling points.
- The strong forces holding ionic compounds prevent them to evaporate easily. Hence, ionic compounds have no smell.
- Solid ionic compounds don’t conduct electricity but they do when they are aqueous or molten. This is because in liquid/aqueous state the ions which conduct electricity are free to move. In solids, these ions are fixed in place.
- Ionic compounds are soluble in water but insoluble in organic compounds. This is because the ions attract water molecules which disrupt the crystal structure, causing them separate & go into solution. Vice versa is when in organic solvent.
Formation of magnesium floride
Magnesium atom loses two
electrons by transferring the electrons to fluorine atoms, one each, making
both stable. The loss of electron forms cation, Mg2+, as it loses 2
electrons, and the gain of electron forms anion, F-. The opposite
charges acquired by both ions attract to each other, forming a strong ionic
bond of MgF2.
Formation of NaCl
Sodium atom loses an
electron by transferring the electron to chlorine atom, making both stable. The
loss of electron forms cation, Na+, and the gain of electron forms
anion, Cl-. The opposite charges acquired by both ions attract to
each other, forming a strong ionic bond of NaCl.
Types of Food additives
- Thickening agents: substances added into food to reduce its amount of water so that the food is thickened. Examples: pectin, acacia gum, gelatin.
- Food dyes (colourings): substances added to food to add or restore the color of food in order to enhance its visual appeal and to match consumer expectations. Examples: Azo compounds such as tartrazine, triphenyl compounds such as brilliant blue FCF. Azo compounds are usually red, orange, brown or yellow in colour while triphenyl compounds are usually blue or green in color.
- Stabilisers: substances added into food to prevent an emulsion from being separated out. Examples: Lecithin, mono- and di-glycerides of fatty acids.
- Flavourings: substances added to food to improve the taste of food and to restore taste loss due to processing. Examples: Sugar, vinegar, common salt, aspartame, monosodium glutamate, synthetic essences such as penthyl ethanoate.
- Antioxidants: substances added to food to prevent oxidation by air that can cause rancid fats and browning of fruits. Examples: ascorbic acid, sodium citrate, tocopherol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT).
- Preservatives: Subtances added to food to slow down or to prevent the growth of microorganisms so that food can be kept for a longer period of time. Examples: sodium benzoate, sulphur dioxide, sodium nitrite, potassium sorbat.