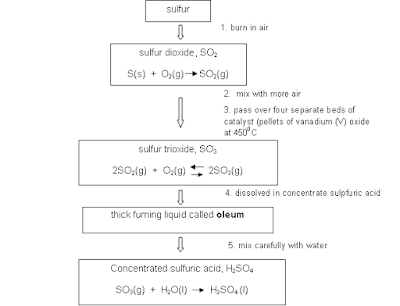
- Both are insoluble in water
- Both are neutral
- Both burn in excess air to produce carbon dioxide and water.
DIFFERENCES:
Alkanes:
- General formula: CnH2n+2
- Saturated hydrocarbon
- Do not decolourise the purple acidified potassium manganate (VII) solution.
- Do not decolourise the reddish-brown bromine solution.
- Burn with a less sooty flame because the percentage by mass of carbon is lower than that of the corresponding alkenes.
Alkenes
- General formula: CnH2n
- Unsaturated hydrocarbon
- Decolourise the purple acidified potassium manganite (VII) solution.
- Decolourise the reddish-brown bromine solution.
- Burn with a more sooty flame because the percentage by mass of carbon is higher than that of the corresponding alkanes.