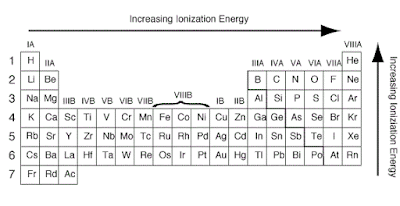
Factors Affecting the Ionization Energy
- Atomic radius: The valence
electrons of an atom with a larger radius experience a less attraction towards
nucleus, hence possesses low ionization energy.
- Effective nuclear charge: The higher the nuclear charge the stronger the
attraction between the nucleus and electrons. This causes the ionization energy
to increase.
- Shielding effect (screening effect):
The shielding effect of the electrons of the inner orbitals causes the outer
electrons to be less attracted to the nucleus and thus decrease the magnitude
of ionization energy.