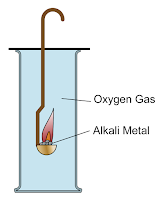
Alkali metals are more
reactive in their reactions with oxygen when going down Group 1. Lithium,
sodium and potassium react with oxygen gas, O2 to form solid metal
oxides with alkaline properties. Alkali
metals react quickly with oxygen and are stored under
oil to prevent oxygen from reaching the surface of the bare metal.
- Lithium burns slowly with a red flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4Li (s) + O2
(g) → 2Li2O (s)
- Sodium burns rapidly with a bright yellow flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4Na(s) + O2 (g)
→ 2Na2O(s)
- Potassium burns very rapidly and violently with a lilac flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4K(s) + O2
(g) → 2K2O(s)