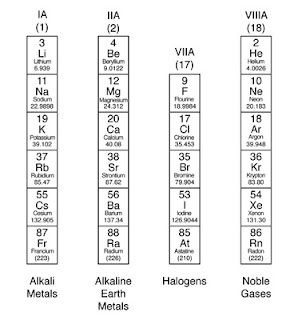
The noble gases, also known
as the inert gases, are located in Group VIII of the periodic table.
The noble gases are helium, neon, argon, krypton, xenon, radon. The
noble gases are relatively non-reactive because they have a complete valence
shell. They have little tendency to gain or lose electrons. The noble gases
have high ionization energies and negligible electronegativities. The noble
gases have low boiling points and are all gases at room temperature.
Summary of Common Properties
·
Fairly nonreactive
·
Complete valence shell
·
High ionization energies
·
Very low electronegativities
·
Low boiling points (all gases at room
temperature)