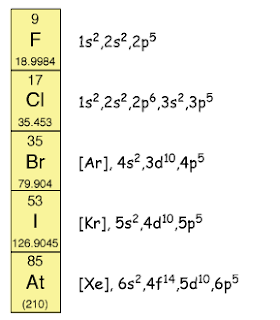
The atomic size of halogen
increases when going down Group 17. Thus, the outermost occupied shell becomes
further away from the nucleus. Hence, the strength of the nucleus to pull
another electron into the outermost occupied shell becomes weaker. Thus,
halogens are less reactive when going down Group 17.