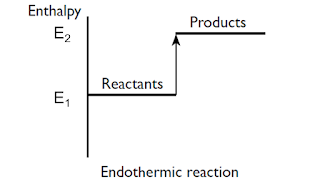
Endothermic reaction
- Endothermic reaction describes
a process which absorbs thermal (heat) energy.
- ∆H = positive
- Products contain more energy
than the reactants. E2 > E1
- Heat energy is absorbed from
the surroundings and is converted to chemical energy during the reaction. This
increases the energy contents of the products.
- Total heat energy given out
during the formation of chemical bonds < Total heat energy absorbed during
the breaking of chemical bonds.
- Temperature of the
surroundings decreases i.e. the reacting mixture becomes cold.