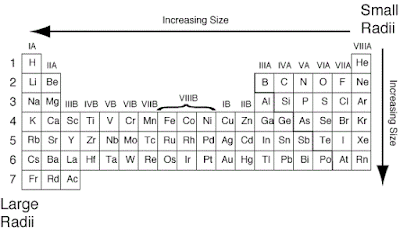
Generally, the atomic radius
decreases across a period from left to right and increases down a given group.
Moving from left to right
across a period, electrons are added one at a time to the outer energy shell.
Electrons within a shell cannot shield each other from the attraction to
protons. Since the number of protons is also increasing, the effective nuclear
charge increases across a period. This causes the atomic radius to decrease.
Moving down a group in the
periodic table, the number of electrons and filled electron shells increases,
but the number of valence electrons remains the same. The outermost electrons
in a group are exposed to the same effective nuclear charge, but electrons are
found farther from the nucleus as the number of filled energy shells increases.
Therefore, the atomic radii increase.