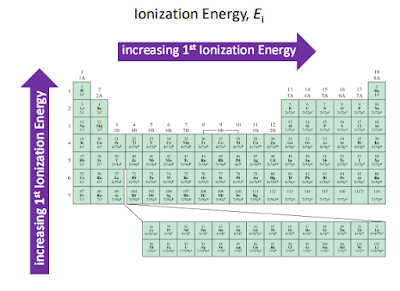
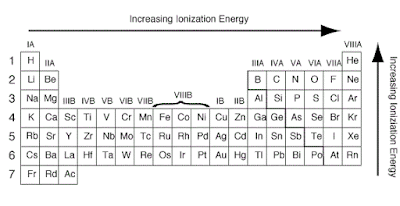
Factors that affect the size of the first ionization energy
The first ionization energy is the energy required to
remove one electron from the parent atom. There are four factors that
affect the size of the first ionization energy:
- The charge on the nucleus
- The distance of the electron from the nucleus
- The number of electrons between the outer electrons and the nucleus
- Whether the electron is alone or paired in its orbital
SPM Form 4: Chemical Formulae and Equations (Checklist)
- Relative atomic mass, Ar, is defined as the mass of one atom of an element relative to 1/12 of the mass of an atom of carbon-12, which has a mass of 12.00 atomic mass units.
- Relative molecular mass, Mr, is defined as the mass of one molecule of an element or compound relative to 1/12 of the mass of an atom of carbon-12, which has a mass of 12.00 atomic mass units.
- Empirical formula is a formula showing the simplest ratio of atoms present.
- Molecular formula is a formula showing the actual number of atoms of each element present in one molecule.
- Chemical equation is a representation of a chemical reaction in words or using chemical formulae.
- The molar mass is the mass of one mole of a sample.
- Molar volume is the volume of one mole of a substance. The molar volume of an ideal gas at STP is 22.4 L/mol.
- Avogadro's constant is the number of particles found in one mole of a substance. Avogadro’s constant=6.0221 x 1023 particles per mole.
- Avogadro’s Law: equal volume of all gases measured under the same conditions of temperature and pressure contain equal numbers of molecules.
SPM Form 4: The Structure of the Atom (Checklist)
- Matter is anything that occupies the space and has a mass. Matter is made up of tiny and discrete particles that are in constant motion. There are spaces between these particles. The tiny particles may be atoms, molecules and ions.
- An atom is the smallest particles of an element that can take part in a chemical reaction.
- A molecule is a group of two or more atoms which are chemically bonded together.
- An ion is a positively-charged or negatively-charged particle.
- Matter consists of small particles that always move and collide among each other.
- The kinetic theory of matter stated the particles move freely when energy is absorb and move slowly when is release, then they are cooled. This theory used to explain the change physical state of matter. The energy used used by the particles to move, we called it kinetic energy.
- Diffusion is the movement of a fluid from an area of higher concentration to an area of lower concentration. Diffusion is a result of the kinetic properties of particles of matter. The particles will mix until they are evenly distributed. Diffusion occurs slowest in solids, faster in liquids and the fastest in gases.
- Melting is the process by which a substance changes from the solid phase to the liquid phase. Melting point is the temperature at which a solid and liquid phase may coexist in equilibrium. The term applies to pure liquids and solutions.
- Freezing is the process through which a substance changes from a liquid to a solid. All liquids except helium undergo freezing when the temperature becomes sufficiently cold. The freezing point is the temperature at which a liquid changes to a solid.
- Condensation is change in the state of matter from the gas phase to the liquid phase.
- Boiling is the process of transition from the liquid state to the gas state, usually occurring when a liquid is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid.
- Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase.
- Isotopes are atoms with the same number of protons, but differing numbers of neutrons. In other words, the have different atomic weights.
- For any atom:
Number of Electrons = Number of Protons
Number of Neutrons = Mass Number -
Atomic Number
Number of Protons = Atomic Number of the Element
SPM Form 4: Acids and Bases (Checklist)
- Acid: A chemical substance which ionizes in water to produce hydrogen ions. An acid will only show its acidic properties when it is dissolved in water. E.g. ethanoic acid CH3COOH only exhibit acidic properties when water is present.
- Alkali: A chemical substance which ionizes in water to produce hydroxide ions. An alkali will only show its alkaline properties when it is dissolved in water. E.g. barium hydroxide, Ba(OH)3 only exhibit alkaline properties when water is present.
- A strong acid is an acid that is completely dissociated in an aqueous solution. E.g. HCl (hydrochloric acid), H2SO4 (sulfuric acid), HNO3 (nitric acid).
- A weak acid is an acid that is partially dissociated in an aqueous solution. E.g. CH3COOH
- A strong base is a base that is completely dissociated in an aqueous solution. E.g. KOH, NaOH
- A weak base is a base that is partially dissociated in an aqueous solution. E.g. NH4OH
- Molarity: The number of moles of solute in 1 dm3 solution. When the molarity of an acid increases, its pH value decreases. When the molarity of an alkali increases, its pH value increases.
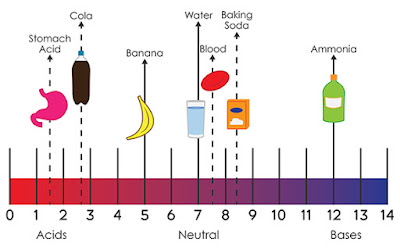
- pH is a measure of hydrogen ion concentration; a measure of the acidity or alkalinity of a solution. Aqueous solutions at 25°C with a pH less than seven are acidic, while those with a pH greater than seven are basic or alkaline. A pH level of is 7.0 at 25°C is defined as 'neutral' because the concentration of H3O+ equals the concentration of OH− in pure water.
- The two factors which determine the pH value of an acid or an alkali are degree of ionization and concentration of the acid or alkali.
- Neutralization: A reaction between an acid and an alkali or a base to produce salt and water.
- Acid-base titration: A quantitative analysis to determine the volume of an acid required to exactly neutralize a fixed volume of an alkali with the help of a suitable indicator.
- End-point: A stage achieved in titration where the volume of acid added exactly neutralizes a fixed volume of an alkali.
Factors Affecting the Ionization Energy
- Atomic radius: The valence electrons of an atom with a larger radius experience a less attraction towards nucleus, hence possesses low ionization energy.
- Effective nuclear charge: The higher the nuclear charge the stronger the attraction between the nucleus and electrons. This causes the ionization energy to increase.
- Shielding effect (screening effect): The shielding effect of the electrons of the inner orbitals causes the outer electrons to be less attracted to the nucleus and thus decrease the magnitude of ionization energy.
Avogradro's Law Definition
Avogadro's Law is the relation which states that at the same temperature
and pressure, equal volumes of all gases contain the same number of molecules.
At constant pressure and temperature, the volume of a gas is directly
proportional to the number of moles of the gas present.
n = number of moles
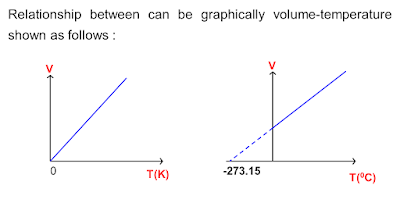
Charles's Law Definition
Charles's Law is an ideal gas law where at constant pressure; the volume of an ideal
gas is directly proportional to its absolute temperature.
where:
Vi = initial pressure
Ti = initial temperature
Vf = final pressure
Tf = final temperature
Vi = initial pressure
Ti = initial temperature
Vf = final pressure
Tf = final temperature
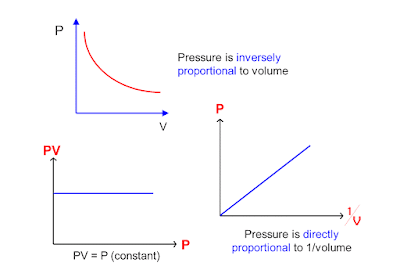
Boyle's law definition
Boyle's Law is an ideal
gas law where at constant temperature; the volume of an ideal gas is
inversely proportional to its absolute
pressure.
PiVi =
PfVf
where
Pi = initial pressure
Pi = initial pressure
Vi =
initial volume
Pf = final pressure
Vf = final volume
Pf = final pressure
Vf = final volume
Alkaloids: Definition and Uses
Alkaloids are a class of organic compounds with nitrogen in a heterocyclic ring. Alkaloids often have
pharmacological effects on humans. Alkaloids are found primarily in plants and
are especially common in certain families of flowering plants. Examples of
alkaloids: nicotine, morphine, cocaine.
- Morphine is a powerful narcotic used for the relief of pain, though its addictive properties limit its usefulness.
- Ergonovine (from the fungus Claviceps purpurea) and ephedrine (from Ephedra species) act as blood-vessel constrictors. Ergonovine is used to reduce uterine hemorrhage after childbirth, and ephedrine is used to relieve the discomfort of common colds, sinusitis, hay fever, and bronchial asthma.
- Cocaine (from Erythroxylon coca) is a very potent local anesthetic.
- Quinine (from Cinchona species) is a powerful antimalarial agent that was formerly the drug of choice for treating that disease, though it has been largely replaced by less toxic and more effective synthetic drugs.
- Two alkaloids, vincristine and vinblastine (from Vinca rosea), are widely used as chemotherapeutic agents in the treatment of many types of cancer.
- Nicotine obtained from the tobacco plant (Nicotiana tabacum) is the principal alkaloid and chief addictive ingredient of the tobacco smoked in cigarettes, cigars, and pipes.
Labels:
Alkaloids,
Cocaine,
Ergonovine,
Heterocyclic ring,
Morphine,
Nicotine,
Quinine,
Vinblastine,
Vincristine
Ionic compounds
An ionic compound is a compound formed by ions bonding together through electrostatic forces. Ionic
compounds form when positive and negative ions share electrons and
form an ionic bond. The strong attraction between positive and negative
ions often produces crystalline solids that have high melting points. Ionic
bonds form instead of covalent bonds when there is a large difference in electronegativity
between the ions.
An ionic bond is formed when one atom accepts or donates one or more of
its valence electrons to another atom.
Example: ionic bond between the sodium and chloride ions in table salt, NaCl.
- Ionic compounds have high melting and boiling points.
- Ionic compounds are mostly soluble in water but insoluble in organic solvents.
- Ionic compounds can conduct electricity in molten state but cannot conduct electricity in solid state.
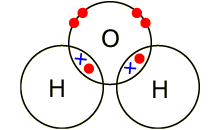
Covalent compounds
A covalent compound is a
molecule formed by covalent bonds, in which the atoms share one or more pairs
of valence electrons. A covalent bond is a chemical link between two atoms in
which electrons are shared between them.
Example: covalent bond between the
oxygen and hydrogen in a water molecule (H2O). Each of the covalent
bonds contains two electrons - one from a hydrogen atom and one from the oxygen
atom. Both atoms share the electrons.
- Covalent compounds have low melting and boiling points.
- Covalent compounds are mostly insoluble in water but soluble in organic solvents.
- Covalent compounds cannot conduct electricity in solid and molten state.
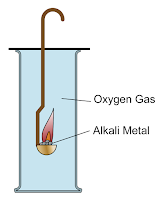
Reaction of Alkali Metals with Oxygen gas
Alkali metals are more
reactive in their reactions with oxygen when going down Group 1. Lithium,
sodium and potassium react with oxygen gas, O2 to form solid metal
oxides with alkaline properties. Alkali
metals react quickly with oxygen and are stored under
oil to prevent oxygen from reaching the surface of the bare metal.
- Lithium burns slowly with a red flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4Li (s) + O2
(g) → 2Li2O (s)
- Sodium burns rapidly with a bright yellow flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4Na(s) + O2 (g)
→ 2Na2O(s)
- Potassium burns very rapidly and violently with a lilac flame. White fumes are produced which become a white solid when cooled to room temperature. The white solid formed dissolves in water to form a colorless solution that turns a red litmus paper blue.
4K(s) + O2
(g) → 2K2O(s)
States of Matter: Changes of State
- Melting is a process solid change to liquid.
- Boiling is a process liquid change to gas
- Condensation is a process gas change to liquid.
- Freezing is a process liquid change to solid.
- Melting points is the temperature at which a solid become a liquid with standard pressure.
- Boiling points is the temperature at which a liquid become a gas with standard pressure.
- Freezing points is the temperature at which a liquid become a solid with standard pressure.
- Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase.
- Deposition is the phase change from gas to solid and is the reverse process of sublimation.
Labels:
Boiling,
Boiling points,
Condensation,
Deposition,
Freezing,
Freezing points,
Matter,
Melting,
Melting points,
Sublimation
SPM Form 4: Introduction to Chemistry (Checklist)
Making observation → Making
inference → Identifying problem → Making hypothesis → Identifying variables → Controlling
variables → Planning an experiment → Collecting data → Interpreting data → Making
conclusion → Writing report
- Fixed variables: the factor that is kept constant throughout the experiment
- Manipulated variables: the factor that is purposely changed in an experiment
- Responding variables: the factor that changes with the manipulated variables
- Hypothesis: a general statement about the relationship between a manipulated variable and a responding variable in order to explain an event or phenomenon.
Acids and Bases Definitions
The most common definitions
of acids and bases are Arrhenius acids and bases, Brønsted-Lowry acids and
bases, and Lewis acids and bases.
Brønsted-Lowry acids and bases
- A Brønsted-Lowry base is a substance that accepts hydrogen ions during a chemical reaction. (Bases are proton acceptors)
- A Brønsted-Lowry acid is a substance that gives up hydrogen ions during a chemical reaction. (Acids are proton donors)
Arrhenius acids and bases
- An Arrhenius acid is a substance that when added to water increases the number of H+ ions in the water. Acid + H2O → H3O+ + conjugate base
- An Arrhenius base is a substance that when added to water increases the number of OH- ions in the water. Base + H2O → conjugate acid + OH-
Lewis acids and bases
- A Lewis acid is a substance that is an electron pair acceptor.
- A Lewis base is a substance that is an electron pair donator.
SPM Form 5: Thermochemistry (Checklist)
- Thermochemistry is the scientific study of heat that is released or absorbed during chemical changes.
- Exothermic reaction: A chemical reaction that involves liberation of heat to the surroundings. The increase in the temperature of the reacting mixture indicates an exothermic reaction.
- Endothermic reaction: A chemical reaction that involves absorption of heat from the. The decrease in the temperature of the reacting mixture indicates an endothermic reaction.
- Heat of precipitation: The change in heat energy when one mole of a precipitate is formed from their ions in aqueous solutions.
- Heat of displacement: The change in heat energy when one mole of a metal is displaced from its salt solution by a more electropositive metal.
- The higher the position of the more electropositive metal is in the electrochemical series, the bigger the value of heat displacement of copper by the metal.
- Heat of neutralization: The change in heat energy when one mole of water is formed from the neutralization of an acid and an alkali.
- Heat of neutralization for reaction between any strong acid and any strong alkali is a constant.
- The values of heat neutralization for reactions between strong acids and strong alkalis are higher than that of reactions between strong acids and weak alkalis, weak acids and strong alkalis or weak acids and weak alkalis.
- Heat of combustion: The change in heat energy when one mole of substance is completely combusted in excess oxygen under standard conditions.
- The greater the number of carbon atoms per molecule of an alcohol, the greater the value of heat of combustion of the alcohol.
What is cathode and anode?
Cathode is
negative terminal or electrode through which electrons enter a direct
current load, such as an electrolytic
cell and the positive terminal of a battery or other source of electrical energy
through which they return. This terminal corresponds in electrochemistry to the terminal at which reduction
occurs. Within a gas discharge tube, electrons travel away from the cathode,
but positive ions (current carriers) travel toward the cathode.
Anode is
the terminal or electrode from which electrons leave a system.
In a battery or other source of direct current the
anode is the negative terminal, but in a passive load it is the positive
terminal. For example, in an electron
tube electron from the cathode travel across the tube toward the
anode, and in an electroplating cell negative ions are deposited at the
anode.
SPM Form 5: Oxidation and Reduction (Checklist)
- Oxidation: A reaction that occurs when a substance gains oxygen.
- Oxidizing agent: The substance that oxidizes other reacting substances in a reaction while it is being reduced.
- Reduction: The reaction that occurs when a substances loses oxygen.
- Reducing agent: The substance that reduces other reacting substances in a reaction in a reaction while it is being oxidized.
- Redox reaction: A chemical reaction in which oxidation and reduction occur simultaneously.
- Metal displacement reaction: A reaction where a more electropositive metal replaces a less electropositive metal from its salt solution. In a metal displacement reaction, a more electropositive metal acts as the reducing agent whereas the ion of the less electropositive metal in the salt solution acts as the oxidizing agent.
- Halogen displacement reaction: A reaction where a more reaction halogen replaces a less reactive halogen from its halide solution. In a halogen displacement reaction, a more reactive halogen acts as the oxidizing agent whereas the halide ion of the less reactive halogen acts as the reducing agent.
- Rusting of iron = the corrosion of ion. Rusting can be prevented when iron is in contact with a more electropositive metal. Rusting occurs faster when iron is in contact with a less electropositive metal.
- Reactivity series of metals: A list of metal that are arranged according to their reactivity with oxygen. The descending order of reactivity of metal with oxygen is magnesium, zinc, iron, lead and copper.
- Carbon is placed between aluminium and zinc in the reactivity series. Magnesium is placed higher than carbon in the reactivity series. Carbon acted as a reducing agent because the metal oxide was reduced to metal in the presence of carbon.
- Hydrogen is placed between zinc and iron in the reactivity series. Hydrogen gas acted as a reducing agent because the metal oxide was reduced to metal in the presence of hydrogen gas.
- In an electrolytic cell, oxidation takes place at the anode whereas reduction takes place at the cathode.
- In a chemical cell, oxidation takes place at the negative terminal (anode) whereas reduction takes place at the positive terminal (cathode).
The melting and freezing points of naphthalene
In the heating of
naphthalene, the heat energy absorbed by the particles is used to overcome the
forces between the particles so that the solid can turn into liquid. In the
cooling curve of naphthalene, the heat loss to the surroundings is exactly
balanced by the heat energy liberated as the particles attract one another to
form a solid. That is why the temperature remains constant for sometime in both heating and cooling curves. The melting and freezing points of naphthalene, C10H8
are 80⁰C.
- Melting point: the temperature at which a solid changes into a liquid at a particular pressure.
- Freezing point: the temperature at which a liquid changes into a solid at a particular pressure.
Reaction of Alkali Metals with Water
Alkali metals are more
reactive in their reactions with water when going down the group. Group 1
elements react with water to form an alkaline metal hydroxide solution and
hydrogen gas, H2.
- Lithium melts and moves slowly at random on the surface of water with plenty of fizz. A colorless solution that turns red litmus paper blue is formed.
2Li (s) + 2H2O
(l) → 2LiOH (aq) + H2 (g)
- Sodium melts and moves rapidly at random on the surface of water with a hissing sound. A colorless solution that turns red litmus paper blue is formed.
2Na (s) + 2H2O
(l) → 2LiOH (aq) + H2 (g)
- Potassium melts moves very fast at random on the surface of water and ignites with a lilac flame, with ‘pop’ and hissing sounds. A colorless solution that turns red litmus paper blue is formed.
2K (s) + 2H2O
(l) → 2KOH (aq) + H2 (g)
The arrangement of the Group
1 elements in ascending order of their reactivity is lithium, sodium and
potassium.
SPM Form 4: Electrochemistry (Checklist)
- Electrode: A conductor in the form of a wire, rod or plate which carry electric current in and out of an electrolyte during electrolysis.
- Electrolyte: A substances that can conduct electricity in molten state or aqueous solution and is decomposed by electric current.
- Non-electrolyte: A substances that cannot conduct electricity in molten state or aqueous solution.
- Anion: A negatively-charged ion.
- Cation: A positively-charged ion.
- Anode: An electrode which is connected to the positive terminal of the source of electricity during electrolysis. (donation of electrons)
- Cathode: An electrode which is connected to the negative terminal of the source of electricity during electrolysis. (acceptance of electrons)
- Electrolysis: The process whereby a compound is broken down into its constituent elements when electricity is passed through an electrolyte.
- Aqueous solution: A solution produced when a compound is dissolved in water.
- Electrochemical series: An arrangement of metals based on the tendency of each metal to donate electrons.
- The lower the position of an ion in the electrochemical series, the higher is the tendency of the ions to be discharged.
- Purification of metals: The process of obtaining a pure metal from an impure metal through electrolysis. E.g. Impure copper can be purified through electrolysis when the impure copper is used as the anode and a pure copper is used as the cathode.
- Electroplating of metals: The process of coating a layer of metal onto another metal using electrolysis.
- Simple voltaic acid: A cell that converts chemical energy to electrical energy. The chemical reactions in a simple voltaic cell produce electricity. No current flow will flow if both electrodes are made of the same metal.
- Electropositivity: A measurement of the ability of an atom to donate electrons to form a positive ion.
- Displacement reaction: A reaction where a more electropositive metal displace another metal from its salt solution. A metal which has a higher position in the electrochemical series is able to displace the metal below it in the series from their salt solutions.
SPM Form 4: Chemical Bonds (Checklist)
- An ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely-charged ions in an ionic compound. Examples: an ionic bond between the sodium and chloride ions in table salt, NaCl.
- A covalent bond is a chemical link between two atoms in which electrons are shared between them. Examples: a covalent bond between the oxygen and hydrogen in a water molecule (H2O).
- Ionic compounds: a compound that consists of positive ions and negative ions arranged in the crystal lattice and are attracted to each other by strong electrostatic forces. Ionic compounds such as magnesium oxide, MgO, sodium chloride, NaCl and iron(III) chloride, FeCl3 can be respectively prepared through a reaction between a metal and a non-metal.
- Covalent compounds are those in which the elements share electrons via covalent bonds.
- Ionic compounds have high melting and boiling point whereas covalent compounds have low melting and boiling points.
- Ionic compounds are mostly soluble in water but insoluble in organic solvents. Covalent compounds are mostly insoluble in water but soluble in organic solvents.
- Ionic compounds can conduct electricity in molten state but cannot conduct electricity in solid state. Covalent compounds cannot conduct electricity in solid and molten states.
- Melting points: the temperature at which a solid turns into a liquid at a fixed pressure.
- Boiling point: the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid.
- Solubility: the maximum quantity of a substance that may be dissolved in another or the maximum amount of solute that may be dissolved in a solvent.
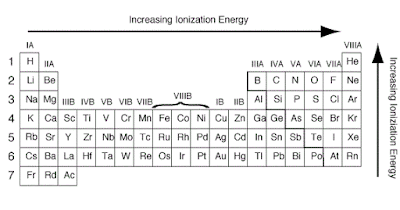
Ionization Energy on Periodic Table
The ionization energy is the
energy involved in removing one mole of electrons from one mole of atoms in the
gaseous state.
Across a period from left to
right, the ionization energy increases. This is due to the increase in atomic
charge having a greater pull on the electrons and therefore more energy is
required to remove electrons.
Going down a group, the ionization
energy decreases. This is due to the outer electrons being further away from
the nucleus and so the attraction is weaker and they are more easily removed.
What is diffusion?
Diffusion is the movement of
particles of a substance in between the particles of another substance. Diffusion
occurs slowest in solids, faster in liquids and the fastest in gases. This is
due to the different arrangement of particles in solid, liquid and gas. The
particles of a solid are packed closely together whereas the particles of a
liquid are packed slightly loose. The particles of a gas are very far apart
from each other and in a random arrangement.
The purple color of
potassium manganite (VII), KMnO4 solution spreads slowly throughout
the water. After a few hours, the water turns uniformly purple.