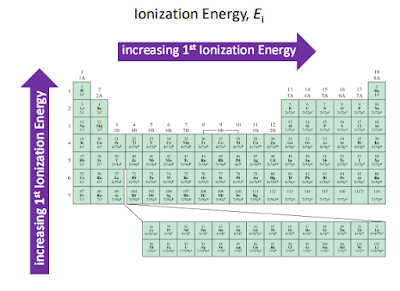
The first ionization energy is the energy required to
remove one electron from the parent atom. There are four factors that
affect the size of the first ionization energy:
- The charge on the nucleus
- The distance of the electron from the nucleus
- The number of electrons between the outer electrons and the nucleus
- Whether the electron is alone or paired in its orbital