Latex is a colloidal solution. It consists of rubber particles dispersed in water. Each rubber particles is made up of many long-chain rubber molecules enclosed by a protein-like membrane which is negatively charged. The negative charges around the rubber particles cause repulsion between these particles to occur when they are near each other. Hence the repulsion between the negatively-charges particles prevents the rubber particles from coming close to each other. Hence latex could not coagulate.
SPM Form 4: Salts (Checklist)
- Salts: An ionic compound formed when the hydrogen ion in an acid is replaced by a metal ion or an ammonium ion. Salts are formed as the product of an acid reaction with an alkali. Soluble salts dissolve in water. Insoluble salts do not dissolve in water.
- Recrystallization: A technique used to purify crystals of a soluble salt by carrying out the crystallization process again on these crystals.
- Precipitation reaction: A reaction which involves the reaction between two aqueous reactants to form an insoluble substance.
- Continuous variation method: An experiment which involves the reaction of a solution at fixed volume and another solution with volumes that varies uniformly.
- All carbonate salts are decomposed on heating to liberate carbon dioxide gas, CO2 except sodium carbonate, Na2CO3 and potassium carbonate, K2CO3.
- All nitrate salts are decomposed on heating to liberate nitrogen dioxide gas, NO2 and oxygen gas, O2 except sodium nitrate, NaNO3 and potassium nitrate, KNO3 which liberate oxygen gas, O2 only.
- The presence of carbonate ions can be confirmed by adding dilute acids.
- The presence of sulphate ions can be confirmed by adding acidified barium chloride, BaCl2 solution.
- The presence of chloride ions can be confirmed by adding acidified silver nitrate, AgNO3 solution.
- The presence of nitrate ions can be confirmed by adding dilute sulphuric acid, H2SO4 followed by iron (II) sulphate, FeSO4 solution and a little concentrated sulphuric acid, H2SO4.
- The identify of cation can be determined by using sodium hydroxide, NaOH solution and ammonia, NH2 solution except aluminium ion, Al3+ and lead (II) ion, Pb2+.
- Ammonium ion can be tested by heating with a strong alkali or adding Nessler’s reagent.
- Iron (II) ion and iron (III) ion can be tested by using potassium hexacyanoferrate (II), K4Fe(CN)6 solution, potassium hexacyanoferrate (III), K3Fe(CN)6 or potassium thiocyanate, KSCN solution.
- Lead (II) ions can be tested by using potassium iodide, KI solution.
SPM Form 4: Manufactured Substances in Industry (Checklist)
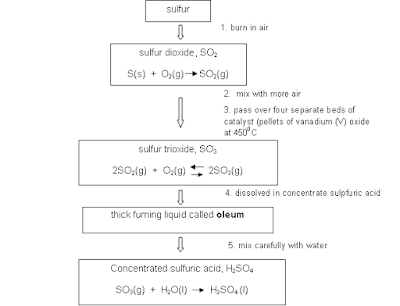
- Sulphuric acid (H2SO4) is a highly corrosive acid made from sulfur dioxide; widely used in the chemical industry.
- Contact process: the industrial process for making sulfuric acid.
- Ammonia (NH3) is colorless, pungent gas composed of nitrogen and hydrogen.
- Haber process: the process for making ammonia from nitrogen and hydrogen, in industry.
- Ammonium fertilizer: A salt that is prepared from the reaction between ammonia and an acid. Ammonium sulphate, (NH4)2 SO4 which is an ammonium fertilizer can be prepared from the reaction between ammonia, NH3 solution and sulphuric acid, H2SO4.
- Alloys: A mixture of two or more elements with certain fixed composition in which the major component is a metal. Alloy is harder than pure metal.
- Metal corrosion: The gradual destruction of a metal by reaction with its environmental. Iron rusts faster than steel. Stainless steel does not rust.
- Polymer: a compound containing very large molecules, formed by polymerization.
- Synthetic polymers are derived from petroleum oil, and made by scientists and engineers. Examples of synthetic polymers include nylon, polyethylene, polyester, Teflon, and epoxy.
- Natural polymers occur in nature and can be extracted and often water-based. Examples of naturally occurring polymers are silk, wool, DNA, cellulose and proteins.
- Glass: A homogeneous material with a random, liquid-like molecular structure.
- Ceramic is a hard, unreactive material that can withstand high temperatures, made by baking clay in a kiln; ceramics are non-conductors.
- A "composite" material is when two or more different materials are combined together to create a superior and unique material.
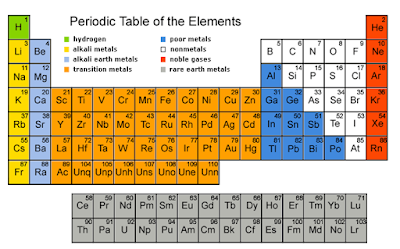
SPM Form 4: Periodic Table of elements (Checklist)
- Periodic Table: the table showing the elements in order of increasing proton number; similar elements are arranged in columns called groups.
- Group: A vertical column of elements in the Periodic table.
- Period: A horizontal row of the Periodic Table; its number tells you how many electron shells there are.
- Alkali metals: the Group I elements of the Periodic Table, which include lithium, sodium, potassium, rubidium, caesium and francium.
- Alkaline earth metals: the Group II elements of the Periodic Table, which include beryllium, magnesium, calcium, strontium, barium, and radium.
- Halogens: the Group VII elements of the Periodic Table, which include fluorine, chlorine, bromine, iodine and astatine.
- Noble gases: the Group 18 elements of the Periodic Table; they are called ‘noble’ or inert gases because they are so unreactive, which include helium, neon, argon, krypton, xenon and radon.
- Transition elements: the elements in the wide middle block of the Periodic Table (elements in group 3 to group 12).
- Metal: an element that shows metallic properties (for example conducts electricity, and forms positive ions)
- Non-metal - an element that does not show metallic properties: the non-metals lie to the right of the zig-zag line in the Periodic Table.
- Amphoteric oxide: An oxide that exhibits both acidic and basic properties.
- The atomic radius is a term used to describe the size of the atom.
- The ionization energy is the energy required to completely remove an electron from a gaseous atom or ion.
- Electron affinity reflects the ability of an atom to accept an electron. It is the energy change that occurs when an electron is added to a gaseous atom.
- Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. The higher the electronegativity of an atom, the greater its attraction for bonding electrons.
SPM Form 4: Chemical Formulae and Equations (Checklist)
- Relative atomic mass, Ar, is defined as the mass of one atom of an element relative to 1/12 of the mass of an atom of carbon-12, which has a mass of 12.00 atomic mass units.
- Relative molecular mass, Mr, is defined as the mass of one molecule of an element or compound relative to 1/12 of the mass of an atom of carbon-12, which has a mass of 12.00 atomic mass units.
- Empirical formula is a formula showing the simplest ratio of atoms present.
- Molecular formula is a formula showing the actual number of atoms of each element present in one molecule.
- Chemical equation is a representation of a chemical reaction in words or using chemical formulae.
- The molar mass is the mass of one mole of a sample.
- Molar volume is the volume of one mole of a substance. The molar volume of an ideal gas at STP is 22.4 L/mol.
- Avogadro's constant is the number of particles found in one mole of a substance. Avogadro’s constant=6.0221 x 1023 particles per mole.
- Avogadro’s Law: equal volume of all gases measured under the same conditions of temperature and pressure contain equal numbers of molecules.
SPM Form 4: Acids and Bases (Checklist)
- Acid: A chemical substance which ionizes in water to produce hydrogen ions. An acid will only show its acidic properties when it is dissolved in water. E.g. ethanoic acid CH3COOH only exhibit acidic properties when water is present.
- Alkali: A chemical substance which ionizes in water to produce hydroxide ions. An alkali will only show its alkaline properties when it is dissolved in water. E.g. barium hydroxide, Ba(OH)3 only exhibit alkaline properties when water is present.
- A strong acid is an acid that is completely dissociated in an aqueous solution. E.g. HCl (hydrochloric acid), H2SO4 (sulfuric acid), HNO3 (nitric acid).
- A weak acid is an acid that is partially dissociated in an aqueous solution. E.g. CH3COOH
- A strong base is a base that is completely dissociated in an aqueous solution. E.g. KOH, NaOH
- A weak base is a base that is partially dissociated in an aqueous solution. E.g. NH4OH
- Molarity: The number of moles of solute in 1 dm3 solution. When the molarity of an acid increases, its pH value decreases. When the molarity of an alkali increases, its pH value increases.
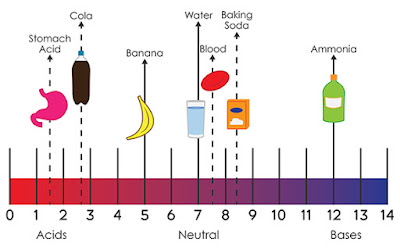
- pH is a measure of hydrogen ion concentration; a measure of the acidity or alkalinity of a solution. Aqueous solutions at 25°C with a pH less than seven are acidic, while those with a pH greater than seven are basic or alkaline. A pH level of is 7.0 at 25°C is defined as 'neutral' because the concentration of H3O+ equals the concentration of OH− in pure water.
- The two factors which determine the pH value of an acid or an alkali are degree of ionization and concentration of the acid or alkali.
- Neutralization: A reaction between an acid and an alkali or a base to produce salt and water.
- Acid-base titration: A quantitative analysis to determine the volume of an acid required to exactly neutralize a fixed volume of an alkali with the help of a suitable indicator.
- End-point: A stage achieved in titration where the volume of acid added exactly neutralizes a fixed volume of an alkali.
SPM Form 4: Introduction to Chemistry (Checklist)
Making observation → Making
inference → Identifying problem → Making hypothesis → Identifying variables → Controlling
variables → Planning an experiment → Collecting data → Interpreting data → Making
conclusion → Writing report
- Fixed variables: the factor that is kept constant throughout the experiment
- Manipulated variables: the factor that is purposely changed in an experiment
- Responding variables: the factor that changes with the manipulated variables
- Hypothesis: a general statement about the relationship between a manipulated variable and a responding variable in order to explain an event or phenomenon.
SPM Form 4: Electrochemistry (Checklist)
- Electrode: A conductor in the form of a wire, rod or plate which carry electric current in and out of an electrolyte during electrolysis.
- Electrolyte: A substances that can conduct electricity in molten state or aqueous solution and is decomposed by electric current.
- Non-electrolyte: A substances that cannot conduct electricity in molten state or aqueous solution.
- Anion: A negatively-charged ion.
- Cation: A positively-charged ion.
- Anode: An electrode which is connected to the positive terminal of the source of electricity during electrolysis. (donation of electrons)
- Cathode: An electrode which is connected to the negative terminal of the source of electricity during electrolysis. (acceptance of electrons)
- Electrolysis: The process whereby a compound is broken down into its constituent elements when electricity is passed through an electrolyte.
- Aqueous solution: A solution produced when a compound is dissolved in water.
- Electrochemical series: An arrangement of metals based on the tendency of each metal to donate electrons.
- The lower the position of an ion in the electrochemical series, the higher is the tendency of the ions to be discharged.
- Purification of metals: The process of obtaining a pure metal from an impure metal through electrolysis. E.g. Impure copper can be purified through electrolysis when the impure copper is used as the anode and a pure copper is used as the cathode.
- Electroplating of metals: The process of coating a layer of metal onto another metal using electrolysis.
- Simple voltaic acid: A cell that converts chemical energy to electrical energy. The chemical reactions in a simple voltaic cell produce electricity. No current flow will flow if both electrodes are made of the same metal.
- Electropositivity: A measurement of the ability of an atom to donate electrons to form a positive ion.
- Displacement reaction: A reaction where a more electropositive metal displace another metal from its salt solution. A metal which has a higher position in the electrochemical series is able to displace the metal below it in the series from their salt solutions.
SPM Form 4: Chemical Bonds (Checklist)
- An ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely-charged ions in an ionic compound. Examples: an ionic bond between the sodium and chloride ions in table salt, NaCl.
- A covalent bond is a chemical link between two atoms in which electrons are shared between them. Examples: a covalent bond between the oxygen and hydrogen in a water molecule (H2O).
- Ionic compounds: a compound that consists of positive ions and negative ions arranged in the crystal lattice and are attracted to each other by strong electrostatic forces. Ionic compounds such as magnesium oxide, MgO, sodium chloride, NaCl and iron(III) chloride, FeCl3 can be respectively prepared through a reaction between a metal and a non-metal.
- Covalent compounds are those in which the elements share electrons via covalent bonds.
- Ionic compounds have high melting and boiling point whereas covalent compounds have low melting and boiling points.
- Ionic compounds are mostly soluble in water but insoluble in organic solvents. Covalent compounds are mostly insoluble in water but soluble in organic solvents.
- Ionic compounds can conduct electricity in molten state but cannot conduct electricity in solid state. Covalent compounds cannot conduct electricity in solid and molten states.
- Melting points: the temperature at which a solid turns into a liquid at a fixed pressure.
- Boiling point: the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid.
- Solubility: the maximum quantity of a substance that may be dissolved in another or the maximum amount of solute that may be dissolved in a solvent.
SPM Chemistry Syllabus Form 4
Chapter 1: Introduction to Chemistry
1.1 Chemistry and its importance
1:2 Scientific methods
Chapter 2: The Structure of the Atom
2.1 Matter
2.2 The atomic structure
2.3 Isotopes
2.4 The electronic structure of an atom
Chapter 3: Chemical Formulae and Equations
3.1 Relative atomic mass and relative molecular mass
3.2 The mole and the number of particles
3.3 The mole and the mass of substances
3.4 The mole and the volume of gas
3.5 Chemical Formulae
3.6 Chemical Equations
Chapter 4: Periodic Table of Elements
4.1 The Periodic Table of Elements
4.2 Group 18 elements
4.3 Group 1 elements
4.4 Group 17 elements
4.5 Elements in a period
4.6 Transition elements
Chapter 5: Chemical Bonds
5.1 Formation of compounds
5.2 Ionic bonds
5.3 Covalent bonds
5.4 The properties of ionic and covalent compounds
Chapter 6: Electrochemistry
6.1 Electrolytes and non-electrolytes
6.2 Electrolysis of molten compounds
6.3 Electrolysis of aqueous solutions
6.4 Electrolysis in industries
6.5 Voltaic cells
6.6 The electrochemical series
Chapter 7: Acids and bases
7.1 Acids and bases
7.2 The strength of acids and alkalis
7.3 Concentrations of acids and alkalis
7.4 Neutralization
Chapter 8: Salts
8.1 Salts
8.2 Qualitative analysis of salts
Chapter 9: Manufactured Substances in Industry
9.1 Sulphuric acid
9.2 Ammonia and its salts
9.3 Alloys
9.4 Synthetic polymers
9.5 Glass and polymers
9.6 Composite materials
Labels:
Acids,
Bases,
Chemical Bonds,
Electrochemistry,
Form 4,
Matter,
Periodic Table,
Polymer,
Salts,
SPM Chemistry