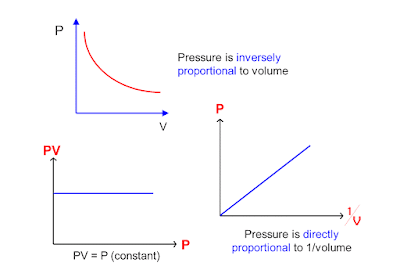
Boyle's Law is an ideal
gas law where at constant temperature; the volume of an ideal gas is
inversely proportional to its absolute
pressure.
PiVi =
PfVf
where
Pi = initial pressure
Pi = initial pressure
Vi =
initial volume
Pf = final pressure
Vf = final volume
Pf = final pressure
Vf = final volume