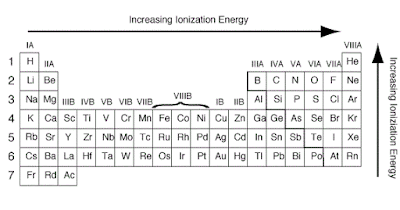
The ionization energy is the
energy involved in removing one mole of electrons from one mole of atoms in the
gaseous state.
Across a period from left to
right, the ionization energy increases. This is due to the increase in atomic
charge having a greater pull on the electrons and therefore more energy is
required to remove electrons.
Going down a group, the ionization
energy decreases. This is due to the outer electrons being further away from
the nucleus and so the attraction is weaker and they are more easily removed.