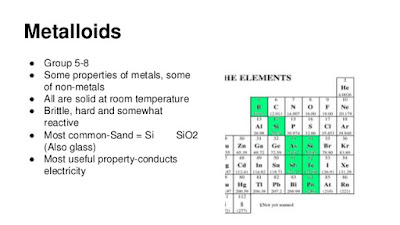
What are metalloids? State the properties of metalloids and list all the metalloids on the Periodic Table.
Metalloids are elements that lie along the zigzag line on the periodic table starting with Boron and moving down in steps. These elements share properties of both metals and non-metals. Metalloids are solids, ductile and malleable. These are good semiconductors. At high temperatures metalloids acts like metals and conduct electricity. At lower temperatures metalloids act like nonmetals and stop electricity from flowing. This property is useful in electronic devices such as computers, tv's and solar cells. The metalloids include: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium.